Medical Necessity, Documentation, Coding, and Billing for Intrathecal Therapy
By Yeshvant A. Navalgund, MD
President and CEO
DNA Advanced Pain Treatment Center
Greensburg, PA
Background
Intrathecal (IT) drug delivery systems are effective for patients with refractory chronic pain, and their use in the United States has been continuously increasing.[1–3] IT therapy provides a valuable option for patients in whom oral or transdermal opioids are ineffective at reasonable doses or cause unacceptable side effects.[4,5] Compared with oral or parenteral routes for pain control, IT delivery uses significantly lower doses of opioids to directly deposit medications near the spinal cord receptors, bypassing the blood-brain barrier, which has led to markedly reduced adverse events.[2,6,7]
“Although it has historically been positioned as a salvage therapy, IT therapy is now being considered earlier in the treatment spectrum.”
Most IT pumps are placed for failed back surgery syndrome, but they are indicated for other types of cancer or noncancer pain as well.[2,3,8] Currently, morphine and ziconotide are the only two agents approved by the Food and Drug Administration for IT analgesia; however, use of other agents is common among pain practitioners.
Quality-of-life measures are improved and overall health care use costs are decreased in selected patients with IT therapy when compared with conventional medical management.[9] Although it has historically been positioned as a salvage therapy, IT therapy is now being considered earlier in the treatment spectrum.[10]
Cost of Therapy
Substantial costs associated with the IT drug delivery system arise at the time of surgical implantation, as well as at the time of revision (eg, pump battery depletion, catheter replacement, device malfunction, infection). A 2015 retrospective study of 365 patients estimated median system longevity to be 5.4 years. Based on 2013 Medicare reimbursement rates, the median cost was $10.46/d. (That cost was only for the hardware and did not include medication and other costs.) The range was $4.08 to $2,973.10 per day, with the highest daily costs resulting from complication-associated premature explants. Because most of the cost is incurred at inception of therapy, the best way to achieve cost savings is by increasing the longevity of the system to average the high initial costs over a long pump life span.[9]
Cost escalation of IT medications with increasing dosage or polyanalgesics during the course of therapy seems to be unavoidable. Polyanalgesia, although more costly (dual therapies average $6.07/d and triple therapies $10.40/d, compared with monotherapy at $2.80/d), is justified based on its effectiveness in restoring pain control. Superior results are achieved when polyanalgesia is initiated early.[11]
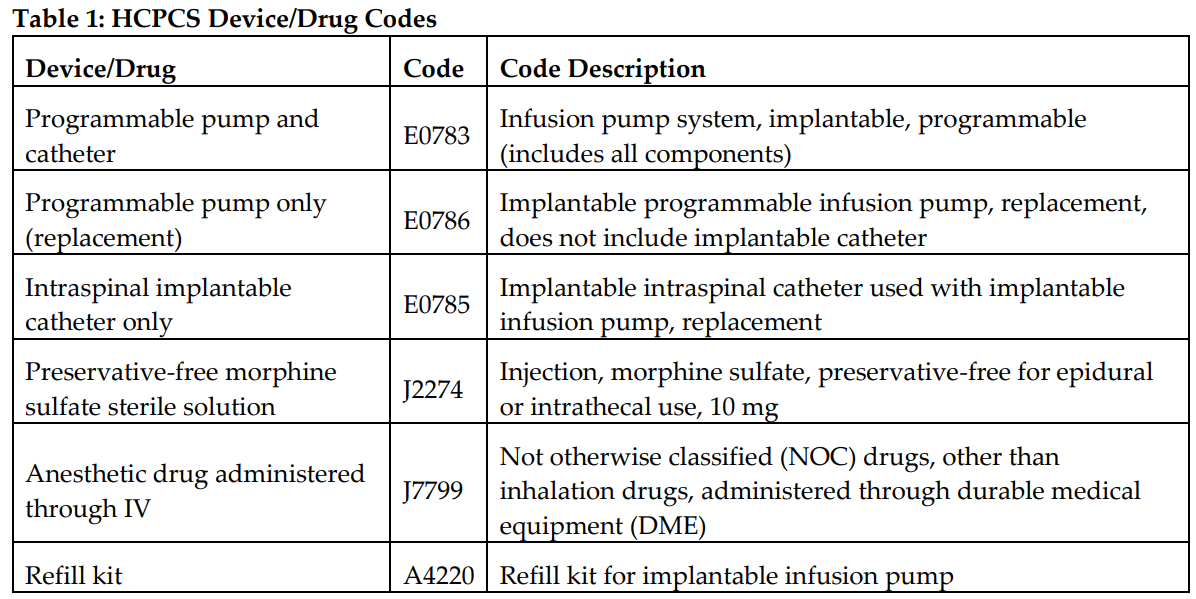
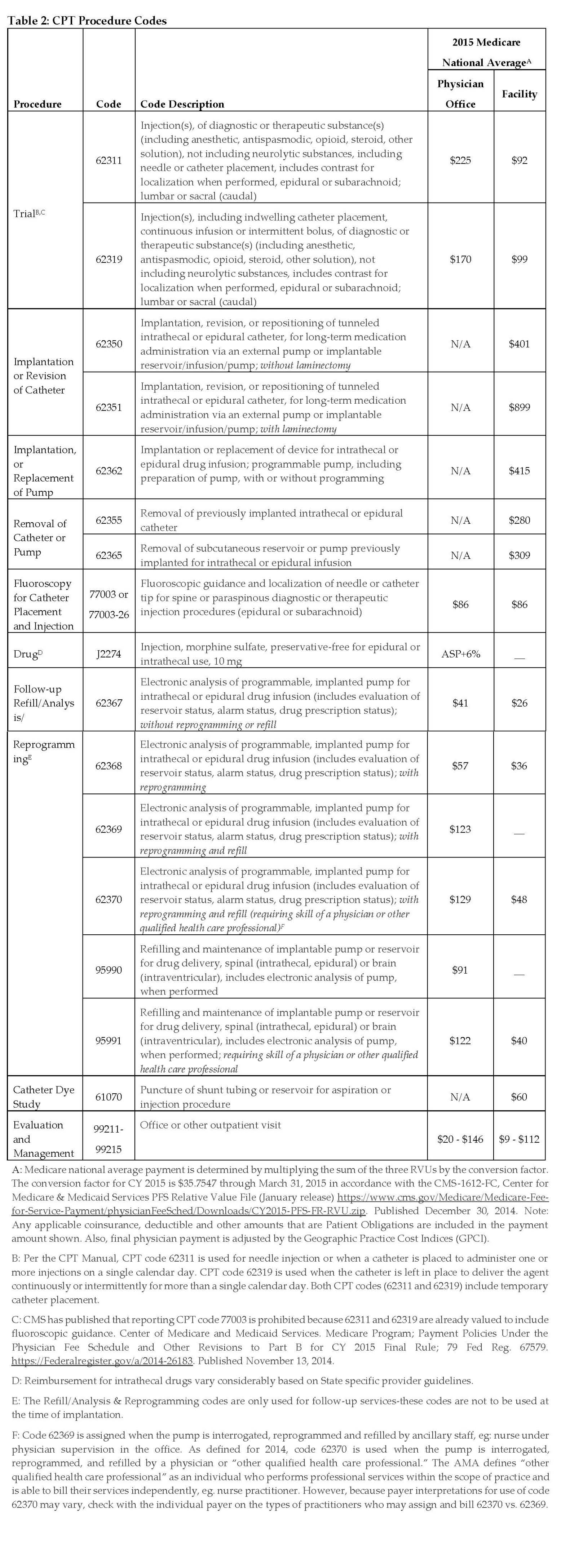
Table 1 provides Health Care Common Procedure Coding System II device and drug codes; Table 2 provides Current Procedural Terminology procedure codes. Different amounts are paid depending on the setting in which a provider rendered the services. “Facility” includes physician services rendered in hospitals and ambulatory surgery centers. Payments are generally lower in the facility setting because the facility is incurring the cost of some of the supplies and other materials. Payments are generally higher in the office setting because a provider incurs all costs there. For refills, analysis, and reprogramming, care should be taken to document the services provided (refill or reprogramming) and who provided them (ancillary staff, mid-level provider, or physician) so that the appropriate billing codes are used.
References
- Deer TR, Prager J, Levy R, et al. Polyanalgesic Consensus Conference 2012: recommendations on trialing for of intrathecal (intraspinal) drug delivery: report of an interdisciplinary expert panel. Neuromodulation. 2012;15(5):420–435.
- Deer TR, Prager J, Levy R, et al. Polyanalgesic Consensus Conference 2012: recommendations for the management of pain by intrathecal (intraspinal) drug delivery: report of an interdisciplinary expert panel. Neuromodulation. 2012;15(5):436–466.
- Paice JA, Penn RD, Shott S. Intraspinal morphine for chronic pain: a retrospective, multicenter study. J Pain Symptom Manage. 1996;11:71–80.
- Smith TJ, Staats PS, Deer T, et al. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain, drug related toxicity, and survival. J Clin Oncol. 2002;20:4040–4049.
- Moore RA, McQuay HJ. Prevalence of opioid adverse events in chronic nonmalignant pain: systematic review of randomized trials of oral opioids. Arthritis Rev Ther. 2005;7(5):R1046–R1051.
- Deer TR, Prager J, Levy R, et al. Polyanalgesic Consensus Conference 2012: recommendations to reduce morbidity and mortality in intrathecal (intraspinal) drug delivery: report of an interdisciplinary expert panel. Neuromodulation. 2012;15(5):467–482.
- Hayek SM, Hanes MC. Intrathecal therapy for chronic pain: current trends and future needs. Curr Pain Headache Rep. 2014;18(1):1–9.
- Turner JA, Sears JM, Loeser JD. Programmable intrathecal opioid delivery systems for chronic noncancer pain: a systematic review of effectiveness and complications. Clin J Pain. 2007;23:180–195.
- Bolash R, Udeh B, Saweris Y, et al. Longevity and cost of implantable intrathecal drug delivery systems for chronic pain management: a retrospective analysis of 365 patients. Neuromodulation. 2015;18(2):150–156.
- Pope JE, Deer TR. Intrathecal drug delivery for pain: a clinical guide and future directions. Pain Manag. 2015;5(3):175–183.
- Kumar K, Rizvi S, Bishop S, et al. Cost impact of intrathecal polyanalgesia. Pain Med. 2013;14:1569–1584.
- Centers for Medicare and Medicaid Services. Physician fee schedule relative value file (January release). December 30, 2014. Available at: https://www. cms.gov/Medicare/Medicare-Fee-for-Service-Payment/physicianFeeSched/ Downloads/CY2015-PFS-FR-RVU.zip. Accessed June 5, 2018.
- Centers for Medicare and Medicaid Services. Medicare program: payment policies under the Physician Fee Schedule and other revisions to Part B for CY 2015 final rule; 79 Fed Reg. 67579. November 13, 2014. Available at: https:// Federalregister.gov/a/2014-26183. Accessed June 5, 2018.
Leave a commentOrder by
Newest on top Oldest on top